DISI Evo – bathless disintegration tester, meticulously engineered for precision, efficiency, and user-friendliness. The device offers a swift solution for disintegration tests, demanding minimal maintenance.

Key benefits:
- For tablets and capsules
- User-friendly touch screen operation
- 100% unattended end-point detection for each tablet
- Fully compliant wireless self-centering baskets
- Automatic test start when target temperature has been reached
- Continuous monitoring of medium level and temperature
- Bathless induction heating allows to bring the water to 37 ˚C for very short heating times
- Modular up to 4 stations
- The system fulfils the requirements of FDA 21 CFR Part 11 (optional).
Real-time monitoring of the remaining height of the tablets
During each upward stroke of the basket, an automatic height measurement tracks the remaining thickness of the tablets. This feature is especially advantageous for coated tablets and capsules with residual shells—no specialized discs needed. It’s also invaluable for R&D labs seeking detailed insights into tablet disintegration characteristics.
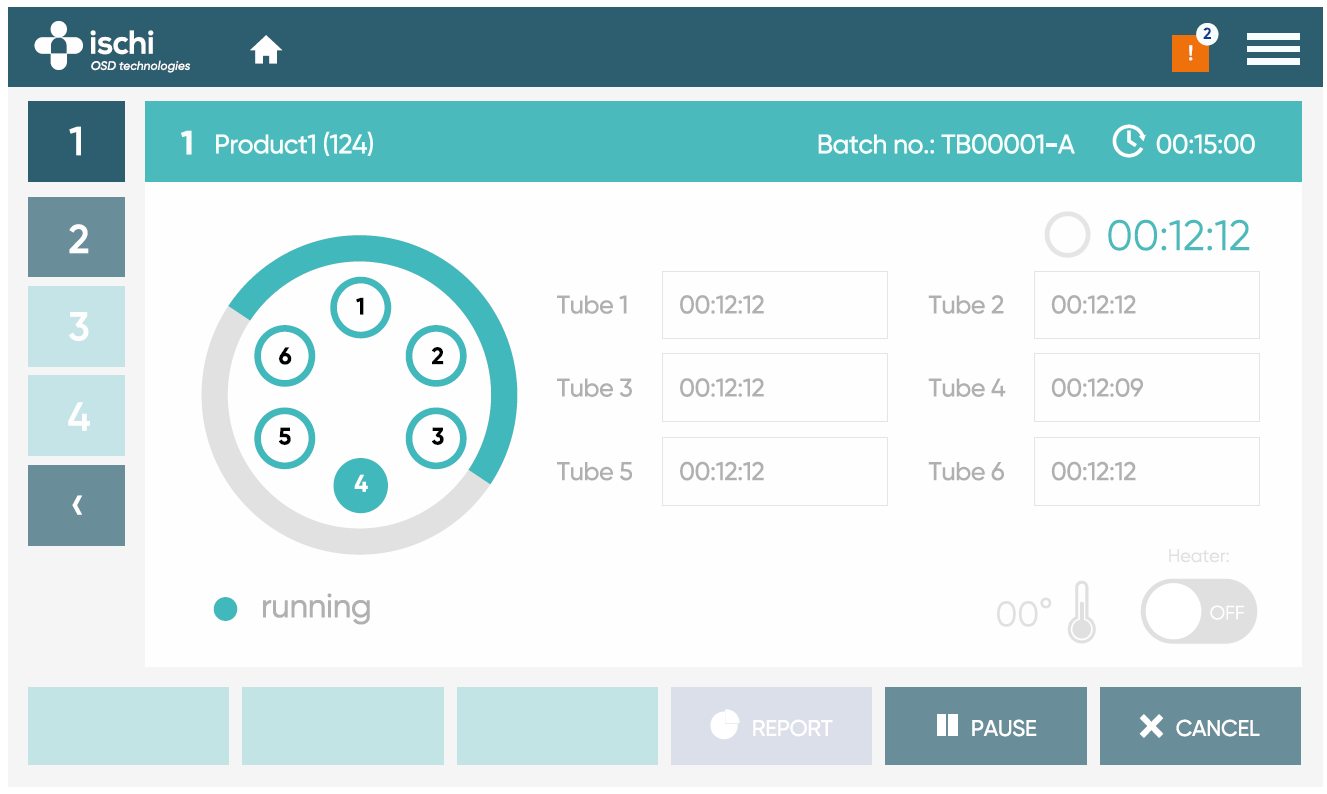

Smart display
Prioritizing user convenience, we’ve streamlined the customer experience to ensure significant time savings in the lab. Our cutting-edge HMI control unit, available as an optional addition, can be conveniently positioned either in front of or on top of the tester. Its high-resolution capacitive display, featuring 1024×600 pixels, delivers sharp contrast and expansive viewing angles, guaranteeing a comfortable and effortless operation.
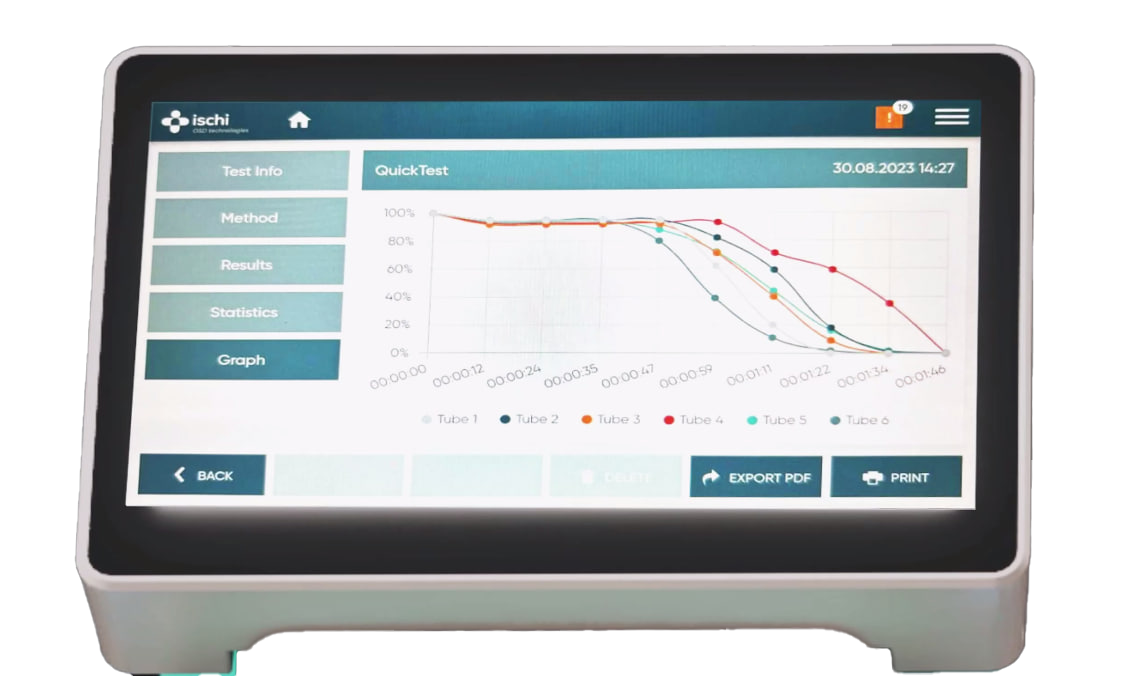
Data analysis
The data analysis concept is based on the successful PH21 production control Software. All data points are stored and may be traced back to the batch and test number. Tabular and / or graphical reports may be printed for single tests and whole batches. It is also possible to print out summary statistics of several batches.
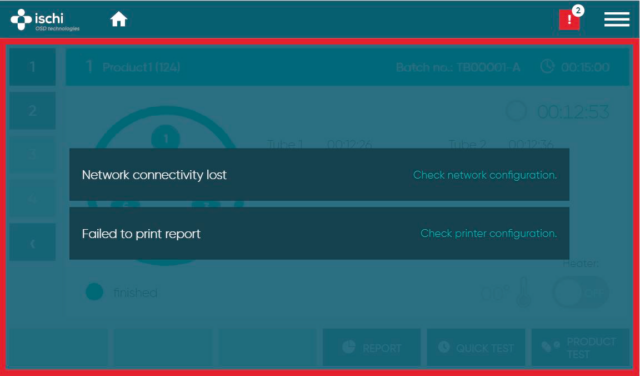
Information center
Our Information Center offers a swift snapshot of all outstanding issues, accompanied by direct links to specific warnings. From expired calibration intervals to unsuccessful exports, it efficiently highlights areas of concern. The Information Center empowers users to promptly address and resolve all challenges from a single window.
Two types of baskets
While using discs for tests, the tablet heights are detected during every upward movement of the basket. The declining height of the tablets is then showcased on the PC or HMI interface. Should the monograph prohibit the use of discs, the operator can manually halt the test. Residual height settings can be individually programmed for capsules and other dosage forms. The innovative measurement principle is based on the attenuation of an electromagnetic field when a short-circuited coil passes through it, a patented method.
A-type disintegration test basket for tablets < Ø 18 mm

B-type for tablets > Ø 18 mm.

Effortless cleaning
With just a few simple steps, you can easily dismantle the testing baskets for cleaning. Not only is the basket assembly remarkably durable, but it can also be effortlessly disassembled and fully submerged in a cleaning bath.


The DISI Evo presents two intuitive operation modes:
Via the Ph21 PC Software on a Windows-based platform. Its familiar user interface ensures rapid user adaptation or Directly using the touch display HMI, offering tactile convenience.Both operational modes are in line with the FDA 21CFRT1 standards, safeguarding data with stringent password protocols. Tailored access control can be set up based on specific requirements. Furthermore, all vital activities are securely recorded in a protected event logbook (audit trail). Operators are kept informed in real-time about tablet heights and receive immediate alerts regarding any unsuccessful tests, like unmet disintegration timeframes.
Data Management
The DISI Evo, boasting a versatile 7-inch touch display, provides an expansive suite of features dedicated to comprehensive data management. Here are its primary standard offerings:
- Storage Capabilities: Infinite storage potential for both products and methods.
- Streamlined Testing: Deactivate and lock any unused products and methods.
- Method Assignments: Link methods to single or multiple products seamlessly.
- Report Access: On-demand calibration and adjustment report printing.
- Data Preservation: Dependable data storage in the durable onboard memory.
- Report Preview: A readily accessible report catalog for data perusal.
- Flexible Reporting: Options to export or print reports, with or without visual graphics.
- Data Export: Effortless report saving, either manual or automated, to USB drives or network folders. Direct PDF export available.
- Data Safety: In-built automatic backup utility with user-defined timing to either USB or network folder.

